When an adduct is formed from Diels Alder reaction of maleic anhydride and furan, what does the adduct decompose to at its melting point?
1 Answer
First, refer to this document for substantiation of what occurs at the melting point.
Now, let's examine the Diels-Alder mechanism for this particular reaction. I have a more general overview here if you want to review what the Diels-Alder reaction is.
DIELS-ALDER MECHANISM
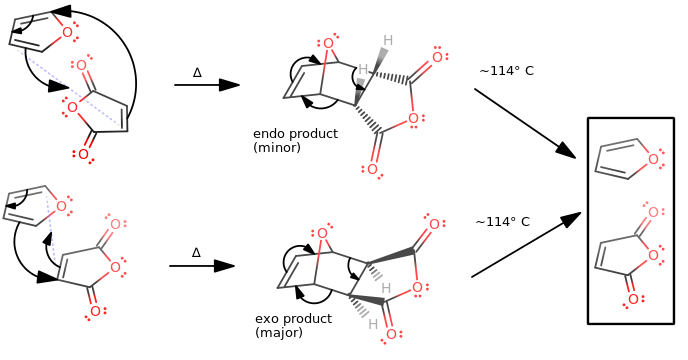
It is a concerted cyclization reaction which generates an adduct of the starting diene (furan) and dienophile (maleic anhydride) that contains two new
The decomposition of this compound at its melting point of approximately
WAIT A MINUTE...
If you have done the lab before where you reacted cyclopentadiene with maleic anhydride, you might wonder, "wait... wasn't the endo product the major product?"
It was. When I did GAUSSIAN calculations for this, I got the
Also, my experimental endo/exo ratio (via integrations on a
So indeed, the endo product for cyclopentadiene + maleic anhydride was kinetically favored (and thermodynamically disfavored) at room temperature.
BUT THIS REACTION...?
But for THIS reaction, of furan with maleic anhydride, as it turns out, the thermodynamic favorability experimentally trumps the kinetic favorability.
The exo product is more thermodynamically stable by about
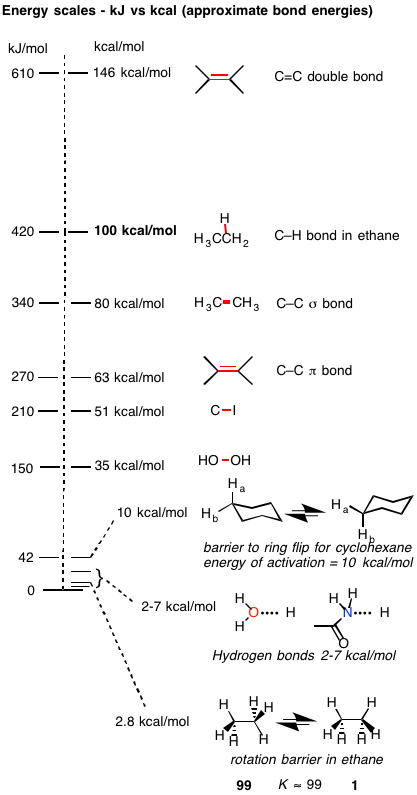
So, the exo product is still the major product. Its thermodynamic stability is actually a smaller difference than the energy barrier to ethane
 http://11452-presscdn-0-51.pagely.netdna-cdn.com/
http://11452-presscdn-0-51.pagely.netdna-cdn.com/

