Which of the following compounds is the most reactive dienophile in a Diels-Alder reaction with 1,3-butadiene, #CH_2=CH-CH=CH_2#?
#CH_2=CH-CH=O#
#(CH_3)_2C=CH_2#
#CH_2=CH-OCH_3#
#CH_3-CH=CH-CH_3#
#CH_2=CH_2#
1 Answer
The most reactive dienophile is propenal.
Explanation:
The Diels-Alder reaction
The Diels-Alder reaction is a [2+4]-cycloaddition reaction between a 1,3-diene and an alkene (a dienophile) to form a cyclohexene.

The transition state involves the overlap of the frontier orbitals — usually the HOMO of the diene and the LUMO of the dienophile.

Nature of the Dienophile
Adding an electron-withdrawing group (EWG) to the alkene lowers the energy of the LUMO.
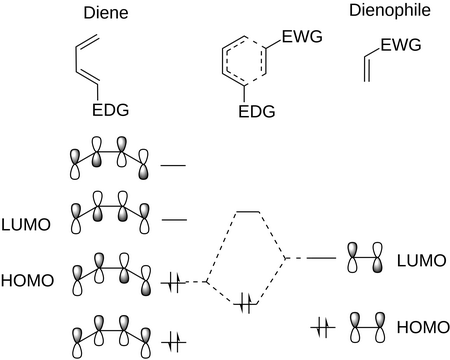
(From Wikipedia)
This decreases the energy gap between the HOMO and the LUMO and causes a dramatic increase in the rate of cycloaddition.
Typical EWGs are carbonyl (
The groups attached to the dienophile in this question are
The most reactive dienophile is the aldehyde — propenal.

