Why does the reaction take place on the central ring of anthracene in a Diels-Alder reaction?
1 Answer
I wonder where you got that, because it's not something I would have thought of asking, and I don't think you are required to know why. Maybe you saw it here.
So sure, let's use that as an example.
Anthracene does apparently react with singlet oxygen (
HOW DID MO DIAGRAMS FOR AROMATIC COMPOUNDS WORK OUT AGAIN?
If you recall, you may have had to draw MO diagrams of benzene, where you counted nodes and eventually came up with the MO diagram. It looked like this.
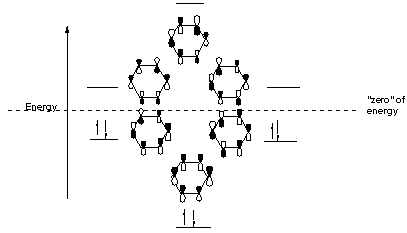
You can see that the highest-energy form of benzene uses the "group orbitals" with the most nodes (three nodal planes).
SO WHY THE CENTRAL RING?
I would hazard a guess that the central
Depictions of a similar reaction can be found here. One of their figures, though small, shows the MOs of anthracene:
Analogizing from the benzene MO diagram above, we can see that the MO configuration of anthracene depicted above resembles the benzene bonding MO configuration on the right (the one with one nodal plane, to the left of the rightmost pair of electrons in the MO diagram).
In this MO configuration:
- The central
#p# orbitals on the leftmost and rightmost rings are the same signs as each other. - The central
#p# orbitals on the central ring are the opposite signs with respect to the aforementioned ones.
Furthermore, we see that in the Diels-Alder mechanism (the first figure in this answer) from an orbital perspective:
- The singlet oxygen donates from a filled
#pi^"*"# antibonding MO (see the MO diagrams at the bottom of the answer) into an#pi^"*"# antibonding MO on anthracene, which belongs to the upper middle carbon on the central ring. - The
#pi# bonding MO belonging to the lower-middle carbon on the central ring back-donates to the other, empty#pi^"*"# antibonding MO (see the MO diagrams at the bottom of the answer) on the singlet oxygen.
That lines up perfectly with the third figure in this answer, and is likely the reason why the central ring is the most reactive one.
IMPORTANT NOTE
That is something that is not easy to justify without calculations, like the usage of Density Functional Theory (DFT) or Hartree-Fock (HF) Theory.
You may want to ask your professor for a second opinion, as this is a question that probably has a very advanced answer. As I had said, without calculations of such things as angle strain and potential energy surfaces using DFT and HF, it is hard to give concrete reasons why.
SO, WHAT'S SINGLET OXYGEN AGAIN?
Singlet oxygen is the excited form of the triplet oxygen we normally we normally think of when we think of the paramagnetic properties of oxygen molecules, so it's no surprise that it's capable of reacting with an aromatic compound.
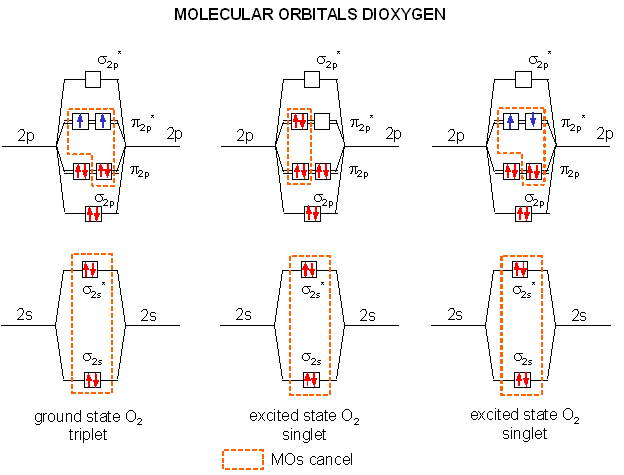
As we see from the image above, it gets the name "singlet" since one of the unpaired electrons (in a
(Normally this is a spin-forbidden state, so it's difficult to achieve this state; therefore, it's unstable and readily reactive.)

