How can I convert a Newman projection for ethane molecule to bond line notation?
1 Answer
So, start with the staggered Newman projection for ethane, or
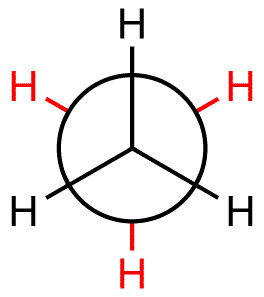
The molecule can have no less than 2 carbon atoms, since Newman projections are made by looking down a bond of carbon atoms. These two carbon atoms that form the bond are drawn as the intersection of the three black lines - this is the front carbon atom - and as the large black circle - this is the back carbon.
As you can see, only hydrogen atoms are attached to these two carbon atoms, so the molecule must obly have 2 carbon atoms.
Now, imagine you're looking down the barrel of a gun. This will represent the plane of the page. For some reason, you've got two propellers attached to the barrel - one closer to you, one further away.
The one closer will represent the front carbon, while the one further away towards the tip of the barrel will be the back carbon.
The atoms you can see coming out of the circle to the left will be going into the plane of the page. The atoms that you see coming out to the right of the circle will be coming out of the plane of the page.
The atoms that point straight down will be coming towards your point of view, while the atoms that point straight up will be further away from your point of view.
Take the front carbon. It has three
The back carbon will also have three
If you draw the two carbon atoms as you'd do for a bond line notation, you'd get a line - this line represents your barrel. Here's how the two attached propellers would look
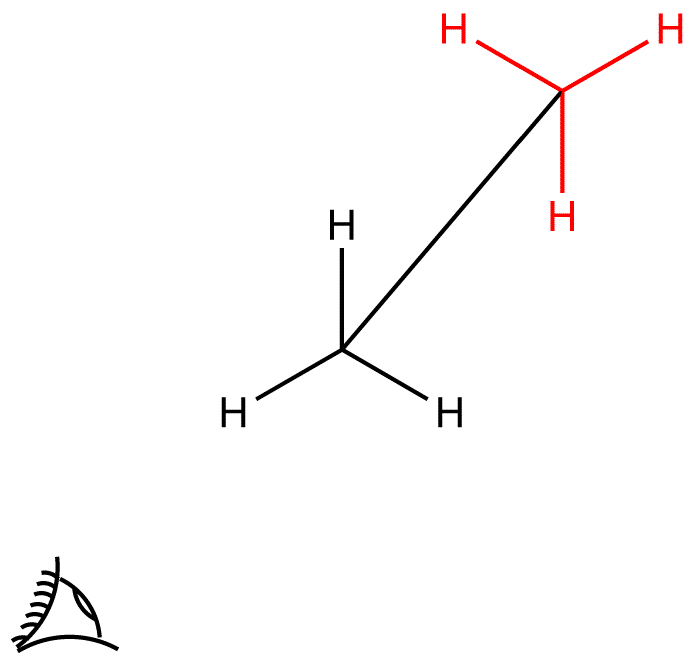
SInce bond line notations don't show the hydrogen atoms attached to carbon atoms, the bond line notation for ethane will just be a straight line

