Question #1ad34
1 Answer
An activator or activating group is a substituent that increases the rate of a substitution reaction on an aromatic ring.
A deactivator or deactivating group is a substituent that decreases the rate of a substitution reaction on an aromatic ring.
The terms are usually applied to electrophilic aromatic substitution reactions such as the nitration of benzene.
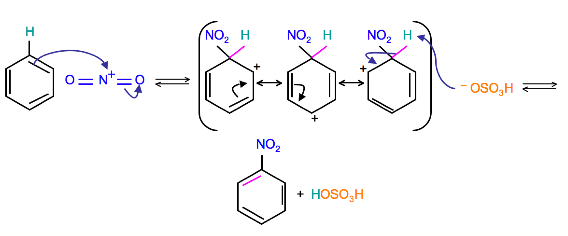
Substituents on the ring change the rate of nitration.
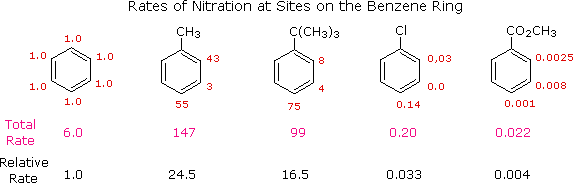
For example, toluene and t-butylbenzene react about 20 times as fast as benzene.
We say that the methyl and t-butyl groups are activating groups.
Similarly, the rate of nitration of methyl benzoate is decreased by a factor of about 250.
We day that the
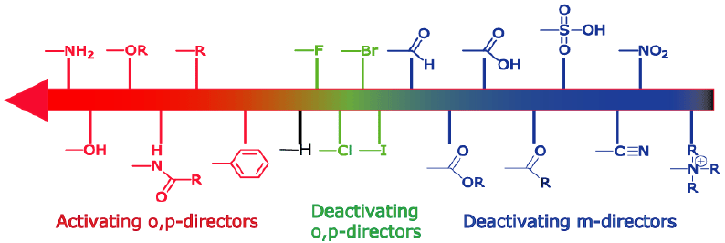
Here's a list of some common activating and deactivating groups.