Question #a2287
1 Answer
May 28, 2015
The base will not remove the nitrile groups — it will convert them to carboxylic acids.
This is not an electrophilic aromatic substitution. It is a Diels-Alder reaction.
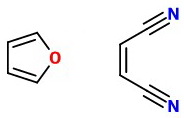
Furan reacts with dienophiles like maleic anhydride to form Diels-Alder adducts.

Both 1,1- and 1,2-dicyanoethene are also excellent dienophiles.
I assume that you are using the 1,2-isomer.
We can imagine it lining up to react with the furan:
The product would be
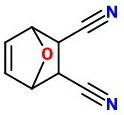
The NaOH hydrolyzes the nitrile groups to carboxylic acids.
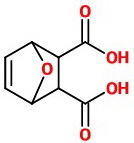
The final product is 7-oxabicyclo[2.2.1]hex-5-ene-2,3-dicarboxylic acid.

