Why is the product of the addition of #Cl_2# to trans-2-butene a meso compound?
1 Answer
Jul 27, 2015
The product is meso because the intermediate involves anti addition to a cyclic chloronium ion.
Explanation:
Both chlorine and bromine react by the same mechanism. Just replace Br by Cl in the diagram below.
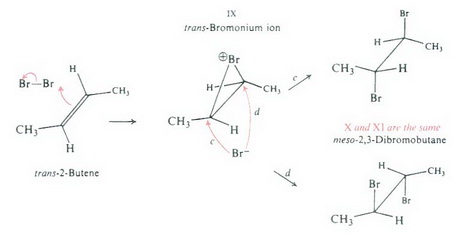
(from www.chemstone.net)
In the first step, the alkene attacks a chlorine molecule to form a cyclic chloronium ion IX.
Now a chloride ion attacks from the bottom of the chloronium ion.
It can attack at either position c or d.
If it attacks at position c, the bond to
If it attacks at position d, the bond to
X and XI are the same meso compound.

