Question #db577
1 Answer
Here's what I got.
Explanation:
So, methylmagnesium bromide,
The reaction will also produce
Here's how the mechanism for this reaction looks like
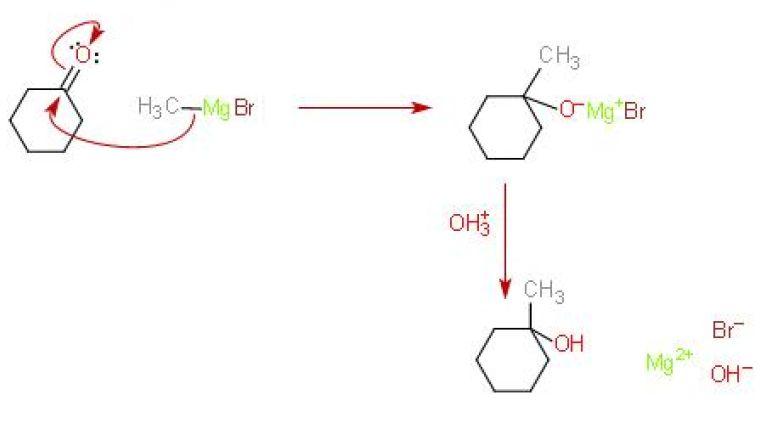
As you know, the difference in electronegativity between carbon and oxygen results in a polar carbon - oxygen bond.
This means that the carbon attached to the oxygen via a double bond, i.e. the one belonging to the carbony group, will be electron deficient.
Grignard reagents act as nucleophiles and attack this electron-deficient carbon, adding across the
Here's where you have a choice for the mechanism. The next step involves the hydrolysis of the intermediary product, which is called a Grignard salt, to form the desired alcohol.
You can do this in one of two ways
- you can add water
- you can add a dilute acid
I think that if you're interested in isolating the resulting alcohol, you should add a dilute acid. I'm not sure about the nature of this salt, by I think that the acid will react with
#"Mg"("OH")"Br" + "H"_3"O"_text((aq])^(+) -> "Mg"_text((aq])^(2+) + "Br"_text((aq])^(-) + 2"H"_2"O"_text((l])#
I chose this option - the weak acid is represented by

