What is the synthetic value of Diels-Alder reaction?
1 Answer
There are quite a few things special about the Diels-Alder reaction.
CARBON-CARBON BONDS ARE GREAT
Thus far, you may have learned perhaps a few one-carbon-carbon bond forming reactions. But have you learned a reaction that forms TWO carbon-carbon bonds? Want to make a ring?
The Diels-Alder is one of them, and it happens in one concerted step!
It's also called a [4 + 2]cycloaddition because it involves four carbons on the conjugated diene and two carbons on the dienophile.
REGIOSELECTIVITY
In addition, the way the molecules are aligned matters. The conjugated diene MUST be s-cis to properly react, because the s-trans conjugated diene has carbons 1 and 4 too far apart to interact with the dienophile in a concerted manner.
Because of the specific way these molecules are aligned, this reaction is very regioselective.
So if
VISUAL CHALLENGE, BUT SIMPLE MECHANISM TO DRAW
Furthermore, it is fairly patterned.
For example... wanna figure out the reactants to make this thing? It's not as painstakingly difficult as you think. Takes time to visualize it, but easy to draw the mechanism---it doesn't really change much from those three concerted arrows.
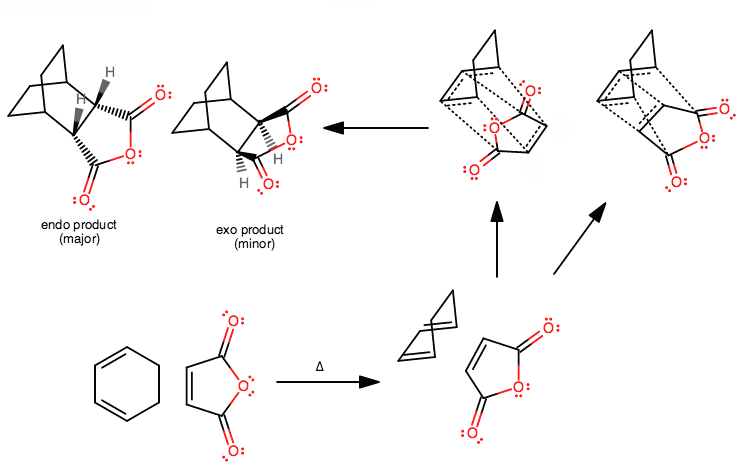
Aside from the transition state (step 3), I think once you understand the Diels-Alder reaction, you should be able to do something like this eventually.
In fact, you might do or have already done something similar to this for a lab, where you react the same anhydride on the right with a cyclopentadiene on the left.
STEREOSELECTIVITY
And of course, there's always the possibility for stereoselectivity; just choose your
You can imagine what you'd get if you had a non-hydrogen substituent on the opposite end of each reactant.

