What are the requirements of the Diels-Alder reaction?
1 Answer
Feb 3, 2016
- Proper alignment of the frontier orbitals (a fancy way of labeling the orbitals that are supposed to interact with each other most easily---the HOMO of the nucleophile and the LUMO of the electrophile) for the conjugated diene and the dienophile). So basically, the molecules gotta be close to each other in a favorable manner.
As a general rule, the positive end of one reactant lines up with the negative end of the other reactant.
- The conjugated diene must be in an s-cis conformation to allow the concerted mechanism to occur. Otherwise carbons 1 and 4 on the conjugated diene are too far away to promote proper frontier orbital overlap with those of the diene.
So, something like this:
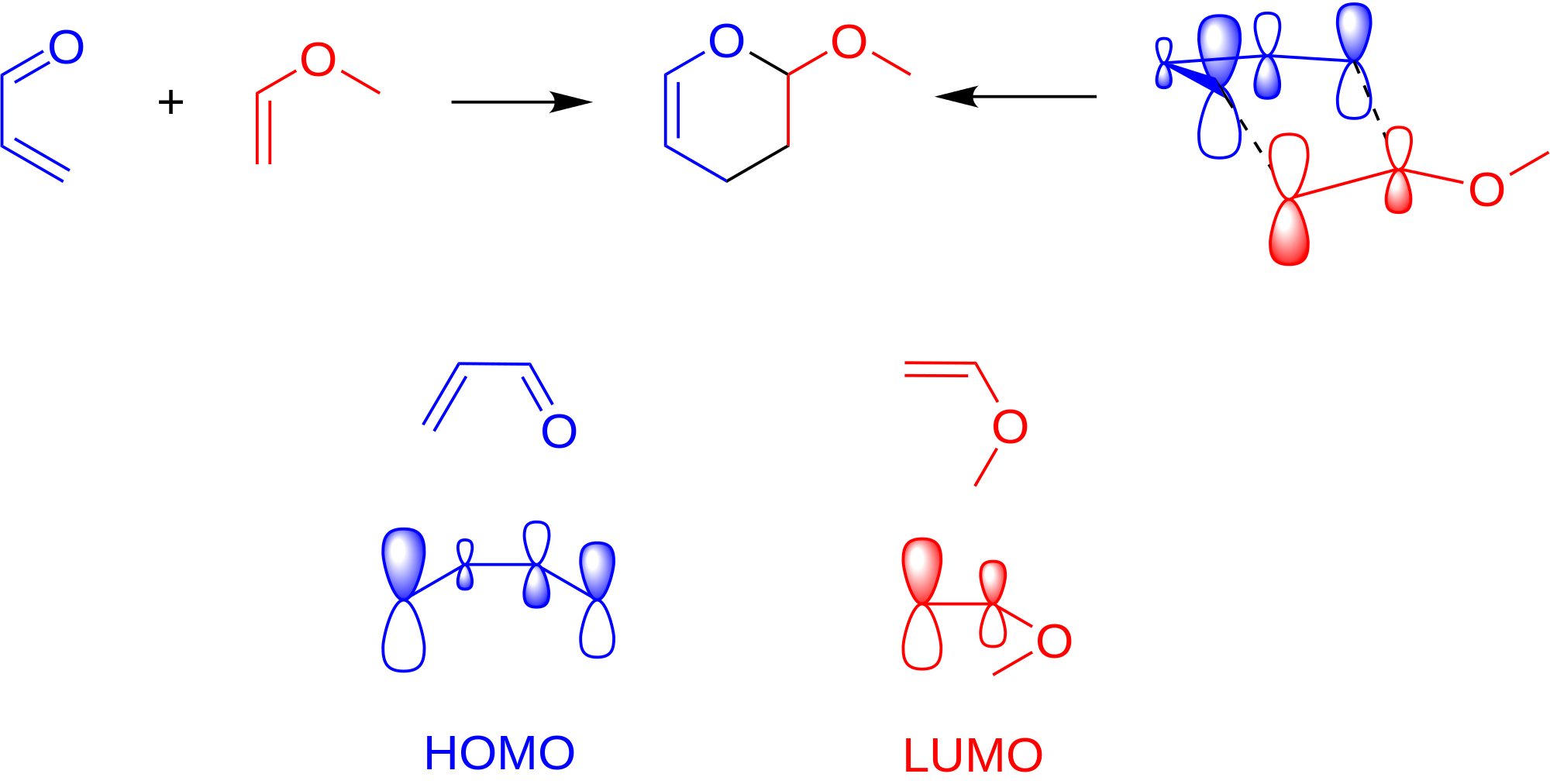
The left reactant is partially negative at the top (the aldehyde group is electron-withdrawing). The right reactant is partially positive at the top (the alkoxide group is electron-donating). Hence, they should align as shown above.
Let's call the bottom carbons of each reactant carbon-1.
- The conjugated diene has an oxygen
#2p_z# that can interact with the#2p_z# on the diene's carbon-2. - The conjugated diene has a carbon-1
#2p_z# that can interact with the#2p_z# on the diene's carbon-1.
I've gone into the reaction itself in detail here:
https://socratic.org/questions/how-does-a-diels-alder-reaction-work

