Is a Diels Alder reaction exothermic?
1 Answer
Feb 4, 2016
Yes, a Diels-Alder reaction is exothermic.
Explanation:
A Diels-Alder involves the reaction of a diene with a dienophile to form a cyclohexene derivative.
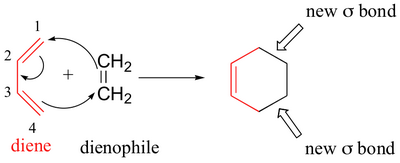
We can use bond dissociation energies (
Typical bond energies are:
This is only a rough value, but it shows that the formation of four new

