What can I do to be able to spot synthesis intermediates and figure out steps more easily?
1 Answer
Not much, really. Mostly practice. Practice your reactions, get your mechanisms to muscle memory, and study with friends.
I can give you three practice examples though on how I would figure one out.
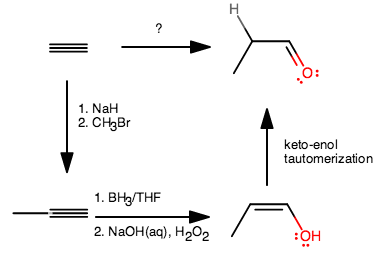
You might think of this in this way:
- The explicit hydrogen is a hint to say that it was just gained. The way it would be gained is from the keto-enol tautomerization.
- What reaction gives a terminal
#"OH"# ? Hydroboration on something because it is anti-Markovnikov. - Why is it an enol? Because that something was an alkyne, not an alkene. Hydroboration of an alkene gives a regular alcohol.
- Why is there an extra carbon? Because it was added.
- How? A nucleophile attacked a
#"CH"_3("LG")# electrophile via#"S"_N2# where#"LG"# is a good leaving group, and#"Br"# is a good leaving group.
Here's how I went about it:
- The first step removes a proton from the alkyne, leaving behind a
#"HC"-="C":^(-)# (acetylide anion). This should be one of the first reactions you learned. That can then attack the#"CH"_3"Br"# in an#"S"_N2# to give the first product. - Hydroboration-oxidation gives the enol (alcoholic alkene), and ends up adding
#"OH"# on the less-substituted carbon (anti-Markovnikov addition). - The enol automatically tautomerizes in base (because you used
#"NaOH"(aq)# ) to give the aldehyde.
Of course, there is more than one way to do this synthesis, but that is one way. Maybe you can find another way? Perhaps involving the generation of that same acetylide anion and addition of the methyl, and then partial reduction of the alkyne, then hydroboration on it, and then oxidizing the result?
And two more examples you can work on to see if you can do them. Perhaps get back to me and see if you had the right idea.
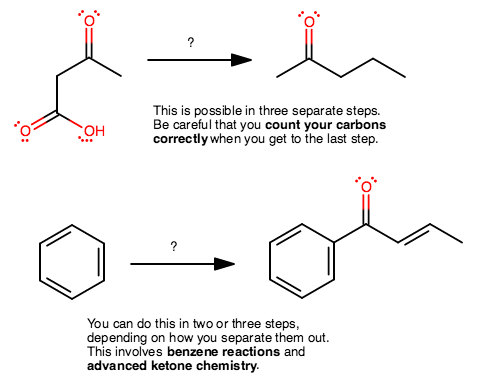
All I do is write out transformations that I know I have done before, and work towards the product step by step. Then I fill in the reactants later.
I would suggest that you do still work backwards, but don't make each individual step too complicated, and that should help you make the association more easily.
So if you wanted to do the problem with the
On the last problem, why is that double bond there, how did the previous intermediate come to be, and how did the ketone group end up on the benzene ring?

