What is the action of 4-methylphenylhydrazine on a carbonyl group, with reaction and example?
1 Answer
In general, we could expect hydrazine to react with a carbonyl group via the Wolff-Kishner reduction mechanism. Let's say we reacted it with acetone. This is typically done in base on high heat.
The end product of a normal Wolff-Kishner reduction is supposed to be the elimination of the carbonyl group from the carbonyl compound, forming its alkane equivalent.
However, notice how one of the nitrogens has only one proton, while normally hydrazine has two on each. I don't think this reaction will utilize the complete mechanism. Here's what I got:
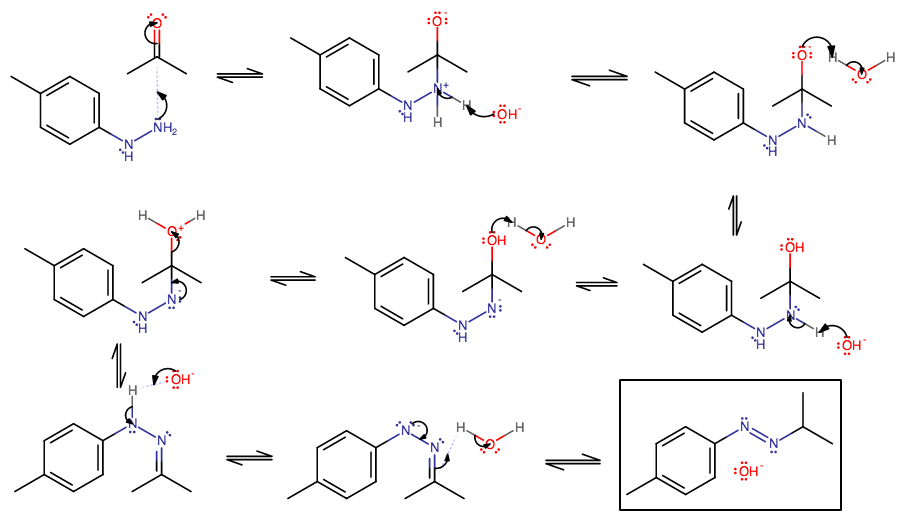
- The nitrogen can attack the carbonyl carbon to start the typical Wolff-Kishner mechanism.
- Proton transfer 1 pt. 1. Eventually that carbonyl group is going to be dehydrated off as
#"H"_2"O"# . - Proton transfer 1 pt. 2.
- Proton transfer 2 pt. 1.
- Proton transfer 2 pt. 2.
- Elimination of that water.
- This is an imine intermediate. There is a proton on the adjacent hydrogen that can be plucked off, but it is facilitated by heat. If you don't add heat, this is a possible end product.
- That encourages the proton acquisition by the imine in this step.
- This is an azobenzene compound, which, without heat or light, isomerizes to the trans form. You may or may not have done a kinetics lab with azobenzene cis-trans light-catalyzed isomerism.
I don't think this mechanism will continue on past this product, because there is no proton on either nitrogen, as there was in step 7, to encourage the generation of another
Now, one might believe that we could still form a diazonium salt, but as it's less stable (and potentially explosive), I would be hesitant to say that it gets produced.
The reaction you might have been taught to form it with is aniline in

