Question #73391
1 Answer
First, let's make sure that we get the amide mechanism.
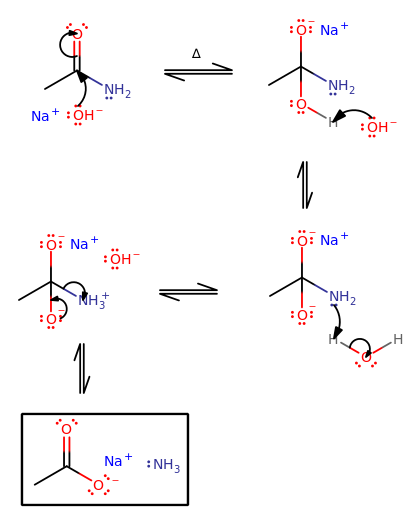
- Heat the amide with
#"NaOH"# , and#"OH"^(-)# can attack the carbonyl carbon, even though it is less electropositive than that of a ketone. The heat helps. - A proton transfer must be made to the
#"NH"_2# because#"NH"_2^(-)# is a terrible leaving group; its pKa is way higher than#36# . On the other hand, the pKa of#"NH"_3# is naturally lower than that of#"O"^(2-)# , so#"NH"_3# can favorably leave in this scenario of high heat. - Finish up the proton transfer.
- Tetrahedral collapse.
And we do indeed form a sodium alkanoate and release
An amine, however, has no electrophilic carbonyl carbon. Instead, the only way it could interact with
Two ways it might go, if at all (ignoring
The amine could grab the proton off of
#"RNH"_2 + "OH"^(-) rightleftharpoons "RNH"_3^(+) + "O"^(2-)#
pKa:#~36# #color(white)()# pKa:#color(white)(aaa)# pKa:#~11#
#color(white)(aaaaaa)# #"Large"#
Or, it could donate a proton to
#"RNH"_2 + "OH"^(-) rightleftharpoons "RNH"^(-) + "H"_2"O"#
pKa:#~36# #color(white)(aaaaaaaaaaaaaaaa)# pKa:#~15.7#
The equilibrium lies on the side of the weaker acid.
On the first one, the protonated amine is a stronger acid than the original amine (lower pKa), so that
On the second one, the amine is the weaker acid in comparison to water, so the
Neither of these is expected to occur. I would not expect a reaction with an amine and

