In a Diels-Alder Reaction, 3-sulfolene produces a 1,3-butadiene and a gas. What is the gas that is produced?
1 Answer
Mar 15, 2016
This is actually a reverse Diels-Alder, also known as a decomposition reaction in general. We know that from how one reactant generates two.
The mechanism for this is fairly intuitive if one realizes that once 1,3-butadiene is to be formed in the mechanism, it will imply breaking the two
If you work through drawing this out, you would see that breaking these bonds will generate
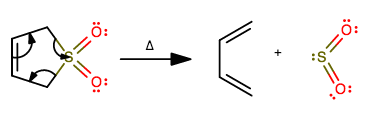
This works because
CHALLENGE: Can you draw the forward reaction? Hint: Sulfur is the source of the donated electrons, instead of a

