What does it mean if the Diels Alder reaction is a concerted reaction?
1 Answer
Mar 22, 2016
It means that all the bonds are formed and broken simultaneously.
Explanation:
Let's begin with something more commonly encountered: Compare the reaction between


The
The Diels-Alder reaction also involves no intermediates. An example of Diels-Alder reaction between 1,3-butadiene and ethene is shown below.
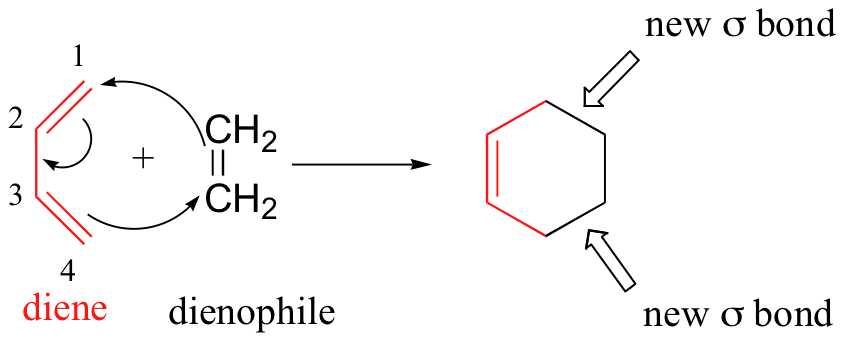
All the electrons move simultaneously. The old bonds are broken at the same time as the new bonds are formed.

