How are alkenes used to make polymers?
1 Answer
Apr 8, 2016
Pressure and heat energy open the double bonds in the alkene and make it bond to form large polymer molecules.
Explanation:
Alkenes are unsaturated hydrocarbons, meaning they don't have all the hydrogen that they could do, because of the double bond between some of the carbons.
Under large amounts of energy - from heat and pressure specifically - the double bond in the alkene can open and bond to other now-opened alkenes in a polymerisation reaction.
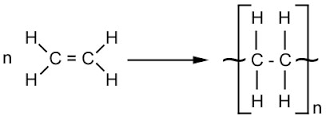
You can see the double bond opening in the image to form polyethene, a common plastic.

