How do you start from benzene to synthesize this aldol condensation product?
2 Answers
How about this?
Explanation:
The retroactive synthesis
The synthesis
Step 1: Friedel-Crafts reaction
Step 2. Aldol condensation
Step 3. Aromatic bromination
Step 4. Preparation of Grignard reagent
Step 5. Grignard addition reaction
Mechanism of aldol condensation
Step 1. Removal of acidic α-hydrogen
Step 2. Nucleophilic attack on a carbonyl group
Step 3. Protonation of the alkoxide
Step 4. Base-catalyzed dehydration of the aldol
As an alternative answer, here is a synthesis plus any necessary mechanisms to go from benzene to your product.
Here is my proposed retro synthesis, first of all.
Now, here's what I think a forward-synthesis could involve, after filling in the reagents. It will involve synthesizing extra stuff from benzene, if you want to start from just benzene.
- A Friedel-Crafts Acylation with acyl chloride gives acetophenone, while a Friedel-Crafts Acylation with phosgene gives benzaldehyde.
- Now, if you use
#"LDA"# (lithium diisopropylamide) as an alternative to a base that contains#"OH"^(-)# , you can force the keto-enolate tautomerization to favor the enolate significantly. -
The catch is that
#"LDA"# doesn't play well with aldehydes (too base-ic), so you'd have to use up all the#"LDA"# first, and then put in the aldehyde afterwards.This generates the
#beta# -hydroxy ketone in the first half of the aldol condensation. If you don't add heat, you should be able to control the reaction to stop here. -
Adding chromic acid, or a suitable oxidizing agent, oxidizes the secondary alcohol to a ketone and stops. It doesn't affect the other carbonyl group because it has no
#alpha# -proton. ;) -
We oxidized the hydroxyl group so that it can be nucleophilically attacked by one equivalent of a methyl grignard reagent, generating the methyl group.
Adding a weak acid (such as
#"NH"_4^(+)# ) works up the reaction to give the hydroxyl group back, but with a methyl attached, and shouldn't significantly protonate the carbonyl group. -
Using
#"LDA"# here with heat is where the aldol condensation comes in. The enolate is generated, and elimination of the hydroxyl group occurs. - Finally, you can separately synthesize a phenyl grignard reagent via aromatic bromination followed by magnesium solid in ether (diethyl ether) to use. This adds that last phenyl group.
A weak acid workup (such as
#"NH"_4^(+)# ) generates the final product without significantly reducing the double bond.
The aldol condensation mechanism using
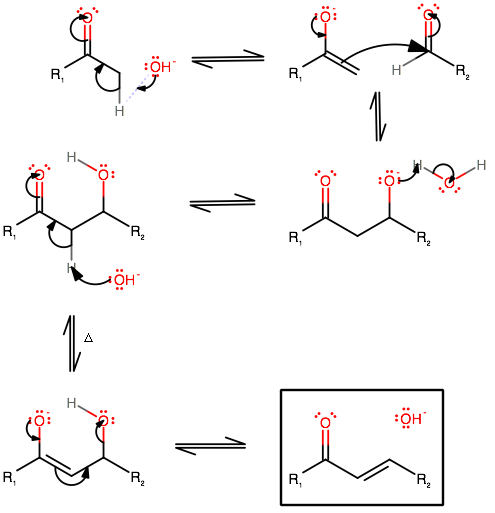
The


