In the reaction of 2-methylcyclopentadiene in #"HBr"#, which product is formed? (Hint: it should be the 1,4-addition product.)
1 Answer
Well, if you consider the bottom-right double bond for resonance stabilization, it should indeed turn out to be the 1,4 addition.
The mechanism of hydrobromination will generate a carbocation intermediate by addition of the
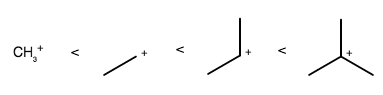
So, you have to determine which one is more stable: tertiary carbocations or secondary carbocations.
Alkyl groups are electron-donating, which stabilize the central positive charge by donating electron density from their
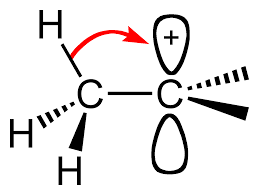
The more alkyl groups surrounding the cationic carbon, the more stabilized it is.
So, the proton will add onto the carbon left of the methyl group to generate the carbocation on the carbon connected to the methyl group. This is consistent with Markovnikov addition.
The righthand double bond is adjacent to the generated cationic carbon, so the
(You were given a hint for 1,4-addition, and as it turns out, we got an addition onto carbons that were 1,4 to each other, clockwise.)