Conversion Methanol to tertiary butyl cyanide?
1 Answer
I will assume you mean t-butyl cyanide, but not t-butyl isocyanide.
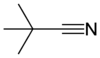
This was a difficult one, and I had to consider whether the original carbon on methanol became:
- the nitrile (
#"C"-="N"# ) carbon - the central tert-butyl carbon
- one of the surrounding tert-butyl carbons
This is ultimately what I came up with:
I ended up choosing one of the surrounding tert-butyl carbons and building the molecule based on that.
- Using
#"PBr"_3# dissolved in pyridine turns an alcohol into an alkyl bromide. - Taking acetylene (
#"HC"-="CH"# ) from a separate process, reacting it with sodium amide (#"NaNH"_2# ) deprotonates one of the protons on acetylene, turning it into a nucleophile. This can backside-attack#"CH"_3"Br"# to generate propyne. - If you use
#"HBr"# on a terminal alkyne or alkene (a hydrobromination), it will react in a Markovnikov fashion, which means the#"Br"# will add onto the more-substituted carbon---the one with less#"H"# atoms. So, both#"Br"# will go onto the middle carbon. - Continue this to generate a geminal dibromide.
-
Here is something you may not have heard of; using methyl lithium or methyl magnesium bromide means you are using an alkyl anion, which is one of the strongest nucleophiles there is.
#"Li"# or#"MgBr"# are both low electronegativity, so the attached carbon is mostly negatively-charged in#"Li"^((+))# #""^((-)):"CH"_3# , for example.Despite the alkyl halide being secondary (
#2^@# ), which is generally borderline in preferring#"S"_N1# vs.#"S"_N2# , the strength of this nucleophile should favor#\mathbf("S"_N2)# displacement of one#\mathbf("Br")# . -
Lastly, adding sodium cyanide would favorably give an
#"S"_N1# reaction to form your product.Since tert-butyl bromide is bulky,
#"CN"^(-)# can't just attack it directly, but it can coordinate with the alkyl halide and wait until the#"Br"^(-)# falls off. It's a slow (rate-determining) step, but it happens, because#"CN"^(-)# is a stronger base than#"Br"^(-)# , favoring#"Br"^(-)# leaving and#"CN"^(-)# adding.

