How can alkenes be used to make ethanol?
1 Answer
Jul 7, 2016
Ethanol is made by the hydration of ethylene in the presence of sulfuric acid
Explanation:
Many simple alcohols, like ethanol, are usually made by the hydration of alkenes in the presence of an acid catalyst.
Ethanol is made by the hydration of ethylene in the presence of a strong acid such as sulfuric acid
The equation below represents the hydration reaction.
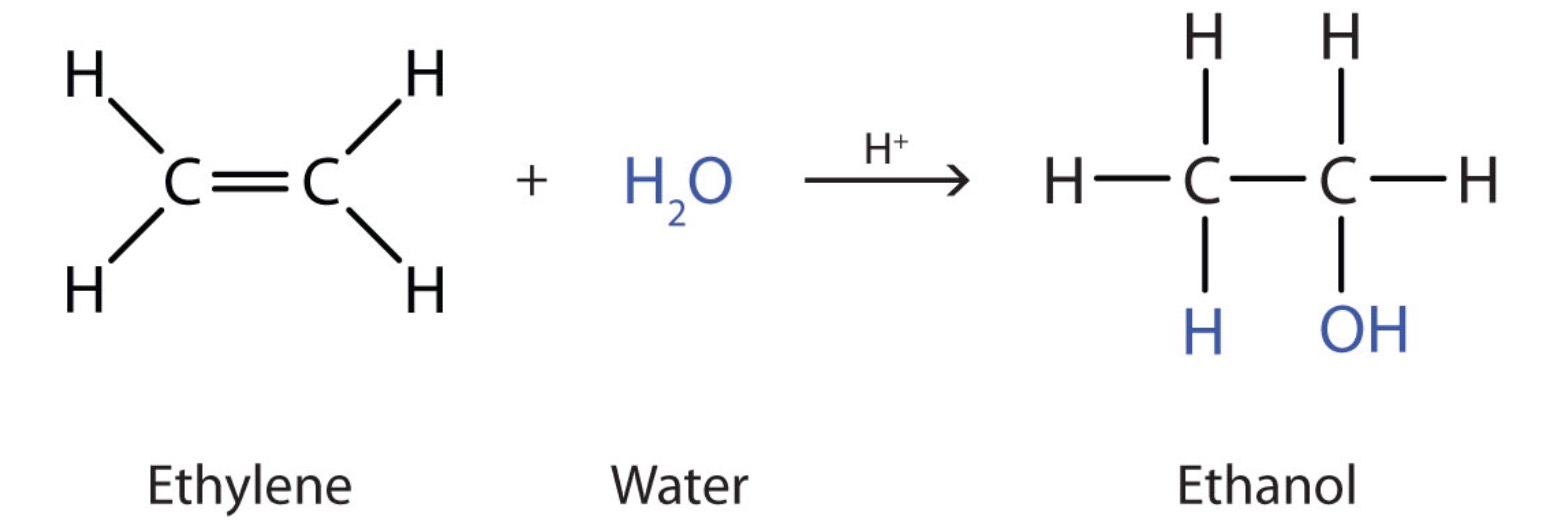
Note that the hydration reaction is an addition reaction - a characteristic reaction of alkenes. In the addition reactions, one of the bonds in the double bond (the
