How do alkenes react with bromine?
1 Answer
Alkenes perturb the electron cloud in
The remaining
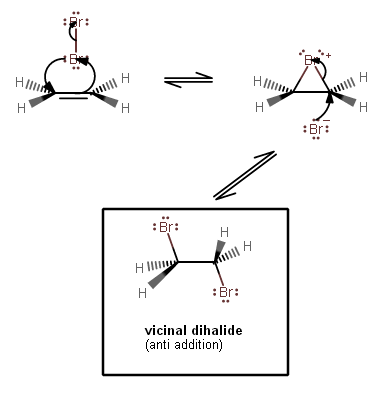
1-1. The
#pi# bonding electrons from the alkene move towards one#"Br"# 's antibonding orbitals; that#"Br"# becomes partially positive (#delta^(+)# ), and the top#"Br"# becomes partially negative (#delta^(-)# ).This accounts for the right-hand arrow.
1-2. Polarizing a bond by perturbing the electron cloud weakens the bond, and in this case, it was enough to break the
#"Br"-"Br"# bond, and the bonding electrons move into now-nonbonding orbitals.This accounts for the upper arrow.
1-3. However, the bottom
#"Br"# also donates electron density into the alkene's antibonding orbitals, thus making a bridging connection between the left and right carbons.This accounts for the left-hand arrow.
That was the complicated part. The rest is not too crazy.
2. The remaining
#"Br"^(-)# is the nucleophile, attacking one of the carbons from the rear (a backside-attack), and breaking one of the bridging bonds (which are weak already).This accounts for both arrows.
The backside-attack is what generates an anti-addition product. This is reflected in the trans relationship of both
In terms of Markovnikov or anti-Markonivkov addition, it doesn't really matter because both

