What is ozonolysis and its applications?
1 Answer
Sep 3, 2016
Ozonolysis is a redox reaction involving
Explanation:
In the presence of oxidising agents, alkenes can turn into alcohols, aldehydes, ketones or carboxylic acids.
In ozonolysis, ozone (
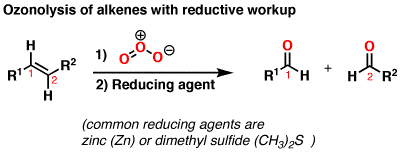
The intermediate formed at the end of the ozonolysis reaction is called an ozonide. To obtain the ketone/aldehyde products, zinc or dimethyl sulfide (reducing agent) is added.
A general overall reaction is shown below.