Can anyone help me with these mechanisms for the formation of spiro heterocycles?
2 Answers
Oct 2, 2016
Okay, I think I've got a plausible mechanism for the second reaction...
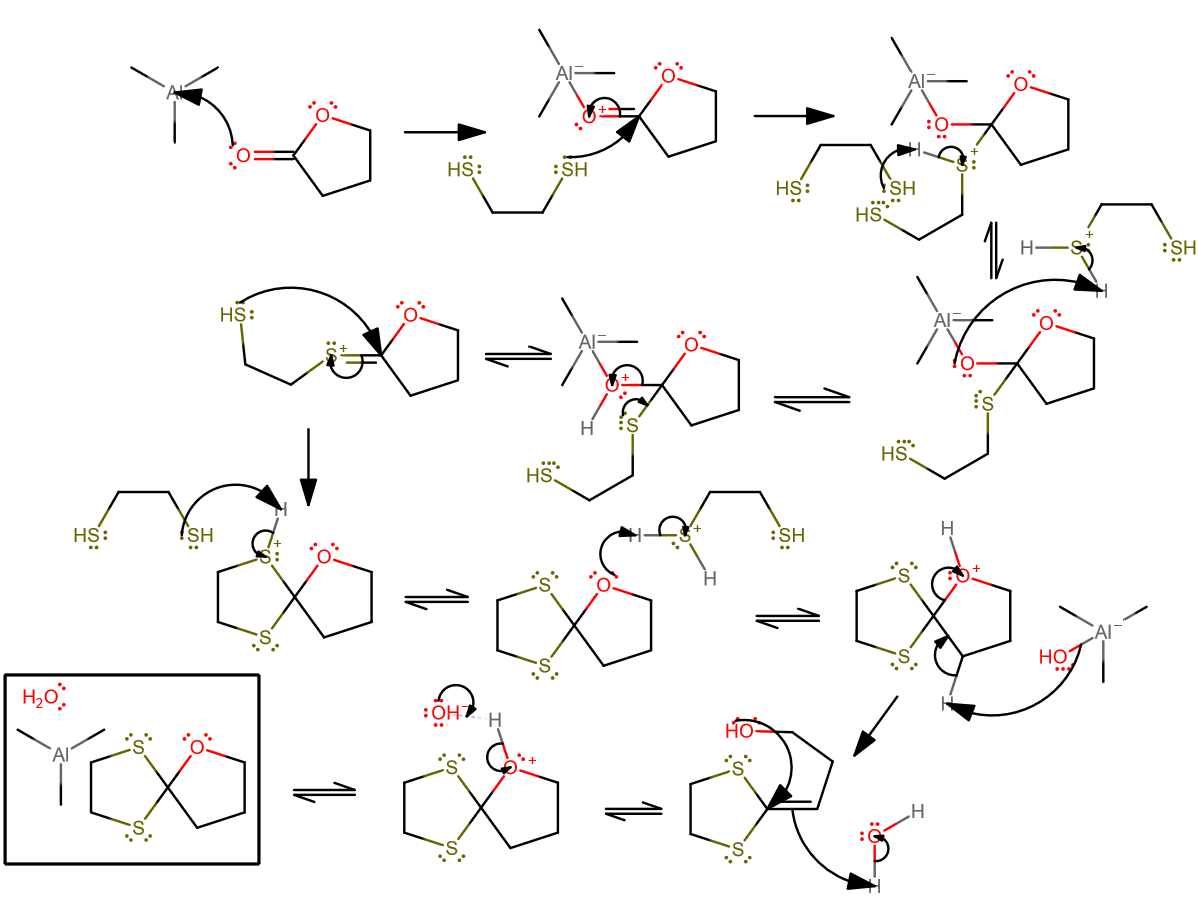
- The trimethylaluminum probably acts similar to how
#"LiAlH"_4# would behave in the presence of ketones, and complexes with the carbonyl oxygen to make it acidic. The three methyl groups though make it less lewis acidic than, say, if they were#"Cl"# . - The ethanethiol can then act as a nucleophile and attach.
- Proton transfer pt1.
- Proton transfer pt2.
- Tetrahedral collapse to form the
#"C"="S"# bond as expected for an intermediate in a protecting group mechanism. - The other end of the thiol can attack now.
- Proton transfer pt1.
- Proton transfer pt2. Even though we've already formed the product, the ethanethiol is not back to how it was when the reaction started.
- Here's where I propose the double bond comes in: while cleaving the
#"C"-"O"# bond via#alpha# -elimination of a proton (compare with enolate formation). - I've kinda condensed two steps into one here, but the first step would have been a
#3^@# carbocation formation via proton donation from the formed water, followed by intramolecular attack. - Finish it off by removing a proton from the oxygen.
Then of course, the trimethylaluminum would react with the water, but we can leave it at that.
The first mechanism is analogous, but easier.
Oct 2, 2016
Here is my proposed mechanism for Part (a).
Explanation:
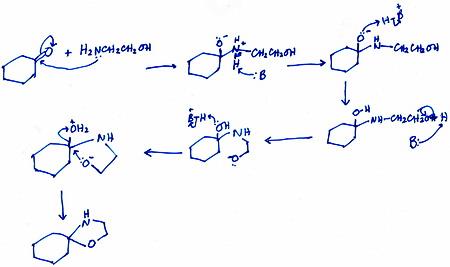
All steps except the first and last are intermolecular transfers.
I used generic species


