Question #d32c3
1 Answer
The final product is 1,1-dimethylpropyl hydrogen sulfate.
Explanation:

It is also unreactive to
Nevertheless, the
Here are the steps for your reactions.
Step 1. Slow ionization of the halide.
This generates an unstable primary carbocation.
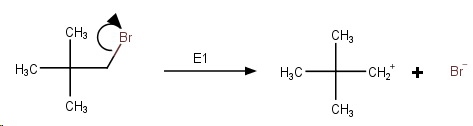
Step 2. The cation rapidly undergoes a methyl shift to form the more stable tertiary carbocation.
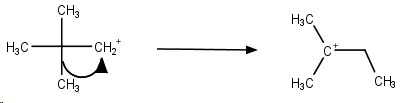
Step 3. The
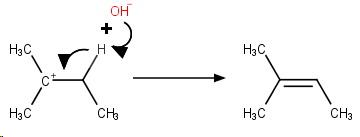
These three steps constitute an
The concentrated sulfuric acid then undergoes electrophilic addition to the double bond.
Step 4. Protonation of the double bond.
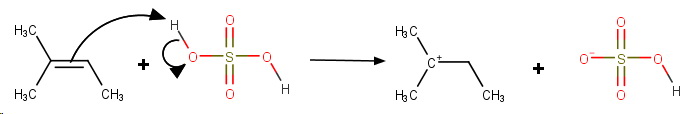
**Step 5. Addition of
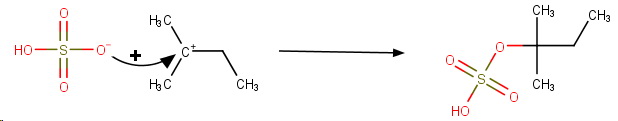
The final product is 1,1-dimethylpropyl hydrogen sulfate.

