Why are alkenes more reactive than other functional groups?
1 Answer
Quick answer: They aren't.
Explanation:
Alkenes are more reactive than some functional groups, but less reactive than others.
It all depends on the functional groups and the specific reactions.
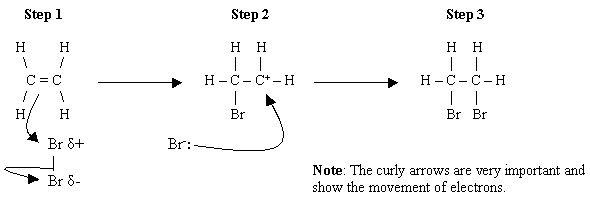
The alkene double bond is reactive because the π electrons are “off to the side” of the two carbon atoms.
They are further away from the two nuclei, so the nuclear attractions are not as strong.

Thus, it is easy for an electrophilic reagent like
However, a functional group like
Thus, only the double bond of 3-chloreprop-1-ene reacts with bromine to give 1,2-dibromo-3-chloropropane.
The π bond is relatively unreactive toward nucleophiles like
In this case, the
Thus, the relative reactivity depends on the reaction and the functional groups you are comparing.