Question #cfa2f
1 Answer
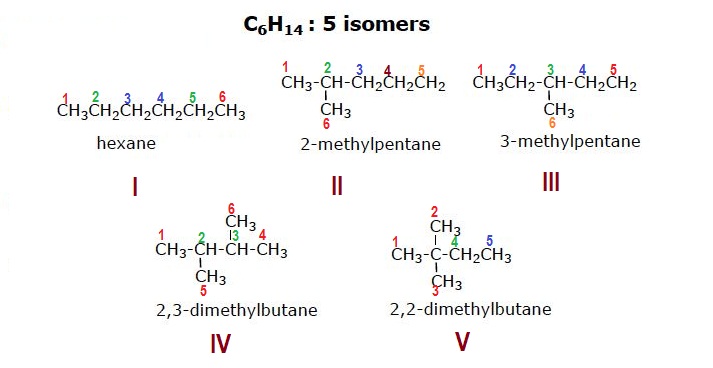
Structural formulae of 5 possible constitutional isomers of the alkane of molecular formula
The 6carbon atoms of each structural formula have been numbered.
In numbering process equivalent carbon atoms in a particular structural formula have been given same color.
We are to find out that isomer of the alkane which yields four constitutional isomers with the formula
So the compound is
In this alkane
Replacement of one H-atom linked with
Replacement of one H-atom linked with
Replacement of one H-atom linked with

