1) The functional group of the compound A of molecular formula #C_2H_4O_2#, widely used as a preservative in pickles is
-COOH
and the compound is ethanoic acid commonly known as acetic acid having structural formula #CH_3COOH#
2) The chemical equation for the reaction with ethanol #(CH_3CH_2OH) # is as follows. The sweet smelling compound B formed here is an ester named ethyl ethanoate and the reaction occurs in presence of conc. #H_2SO_4#
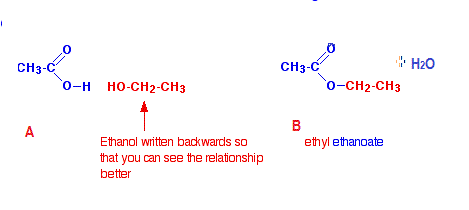
3)The Compound A can be reproduced from B by hydrolysis
4) The name of the process is hydrolysis . In this process B is heated with aqueous solution of #NaOHorKOH# and the ethanol produced escapes as volatile matter. As a result a solution of sodium ethanoate is obtained. This solution on heating with . #H_2SO_4# solution gives back the ethanoic acid.
Equations may be written as follows
#CH_3COOCH_2CH_3 +NaOH->CH_3COONa+CH_3CH_2OH#
#CH_3COONa+ H^+ ->CH_3COOH + Na^+ #


