What is the product of the reaction between 2-bromo-2-methylpentane and sodium ethoxide in ethanol?
1 Answer
Mar 19, 2017
Here's what I get.
Explanation:
The tertiary substrate favours an
The strong base favours elimination, so we draw an
The strongest base in the reaction mixture is the ethoxide ion, formed by the reaction
The mechanism is
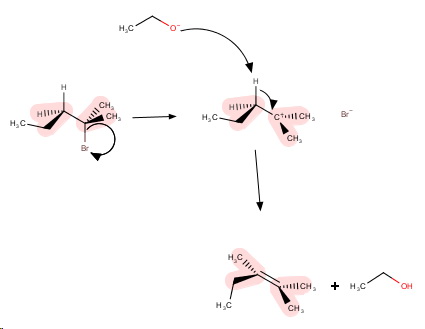
Step 1. The
Step 2. The strong base removes a β-hydrogen atom to form the most stable (most highly-substituted) alkene.

