What are the products when glycine, β-alanine, γ-aminobutyric acid are heated?
1 Answer
Mar 26, 2017
Here's what I get.
Explanation:
An important principle is that if two atoms are in a 1,5- or a 1,6-relationship to each other and can react, they will almost certainly do so.
This is because
- the two groups can easily come close to each other during normal bond rotations within the molecule and
- the new 5- or 6-membered ring is almost strain-free
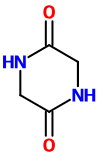
Glycine (α-aminoacetic acid)
Thus, when glycine is heated to about 260 °C, it condenses to form the stable 6-membered ring structure of piperazine-2,5-dione.
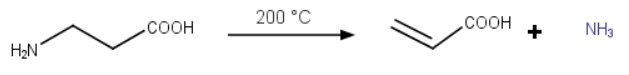
β-alanine (β-aminopropionic acid)
β-Alanine cannot easily form a 5- or 6-membered ring.
Instead, it loses a molecule of ammonia when heated to 200 °C and forms a stable α,β-unsaturated carboxylic acid.
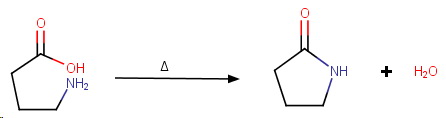
γ-Aminobutyric acid
γ-Aminobutyric acid is easily dehydrated to form a stable 5-membered lactam.