Question #c606f
1 Answer
The only intermolecular forces in cyclohexane are London dispersion forces.
Explanation:
You are probably used to seeing the structure of cyclohexane drawn like this.

The structure is more like this.

The electron clouds fill up much of the space, so the molecule resembles a flat disk.
The only bonds in cyclohexane are nonpolar
Hence, there are no dipole-dipole or stronger intermolecular forces.
There are only weak London dispersion forces.
These arise because the electron clouds are constantly fluctuating.
At a given instant, there might be a little less electron density in one part of the molecule.
The slight positive charge will attract the electrons in a neighbouring molecule.
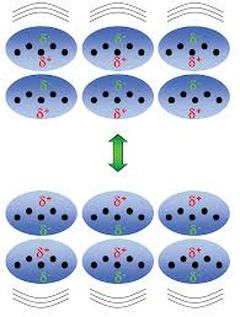
An instant later, the temporary charge imbalance will be in another part of the molecule.
Thus, London dispersion forces are relatively weak.
Cyclohexane doesn't even freeze until the temperature has dropped to 6.5 °C.

