Question #323a9
1 Answer
Activation energy
Explanation:
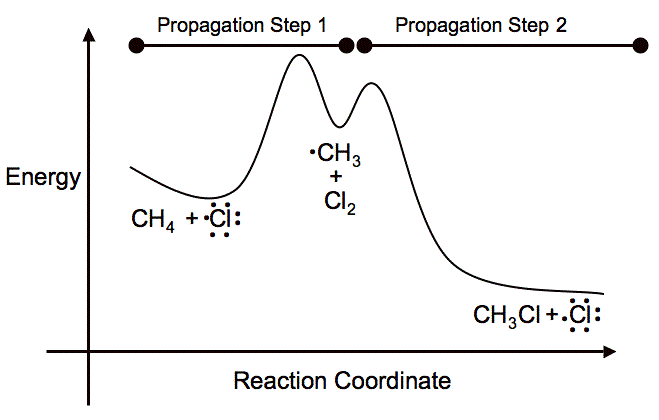
The activation energy for the chlorination of methane is very high at room temperature, so you have to either use heat or light to initiate the reaction between
The first propagation step forms a methyl carbocation. This reaction results in a positive
It is just getting past the energy barrier that you need to put in a lot of energy for the reaction to occur. Overall, the reaction is exothermic, but if you look solely at the initiation and first propagation steps, you end up with an endothermic reaction.
Here is the potential-energy diagram for the reaction to visually show what I explained.