Fischer Projection to Bond Line View
Key Questions
-
Answer:
You draw a zig-zag line with the correct number of carbon atoms, then you attach the groups in the correct locations.
Explanation:
For example, the Fischer projection of D-gulose is

To convert the Fischer projection to a bond line formula you just draw a zig-zag line of six carbon atoms.
Then you put an aldehyde group at
#"C-1"# and an#"OH"# group on each of the other five carbon atoms.You get

(from www.chemeddl.org)Note that a bond line formula gives no stereochemical information.
It shows only which atoms are connected to the other atoms.
The structure above could represent any of the fifteen stereoisomers of D-gulose.
-
Answer:
It is useful to convert Fischer projections to bond line views when you want to show the basic structure of the molecule without any stereochemical information.
Explanation:
For example, the Fischer projection of D-gulose is

To convert the Fischer projection to a bond line formula you just draw a zig-zag line of six carbon atoms.
Then you put an aldehyde group at
#"C-1"# and#"OH"# groups on each of the other five carbon atoms.You get

Note that the bond line formula gives no stereochemical information.
It shows only which atoms are connected to the other atoms.
The structure above could represent any of the fifteen stereoisomers of D-gulose.
-
Answer:
A bond-line view is a condensed way of representing the structural formula of a molecule.
Explanation:
For example, here is the structural formula of retinol, a compound that is important in the chemistry of vision.
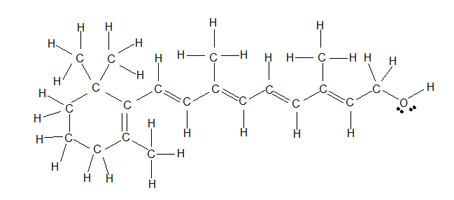
It takes a long time to write out all these atoms and bonds, so chemists have developed a shorthand method called a bond-line view.
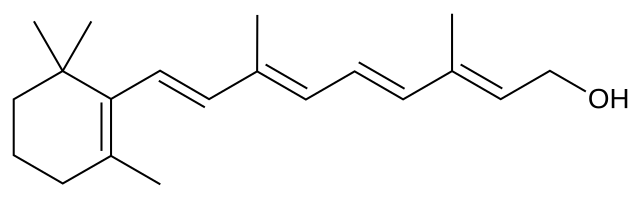
The rules for drawing bond-line structures are:
- The carbon atoms and the hydrogen atoms attached to them are not shown.
- Only the bonds between the carbon atoms are shown as lines.
- The vertices and end of lines represent carbon atoms.
- Any unfilled valences on carbon are assumed to be filled by hydrogen atoms.
- All atoms other than carbon, plus any hydrogen atoms attached to them, are shown.
- Exception: The hydrogen in an aldehyde group is usually shown.