What happens if you mix acetone and chloroform?
1 Answer
This is very unusual, but I will say hydrogen-bonding. Acetone weakly hydrogen-bonds with chloroform. (When I say weakly, I mean weaker than you would see in typical examples like
As a review, hydrogen-bonding is when we have either of the following:
- the hydrogen on an electronegative atom has electron density that is getting pulled on by an electronegative atom nearby.
- a particularly electropositive hydrogen atom has electron density that is getting pulled on by an electronegative atom nearby.
For acetone and chloroform, we have the second option.
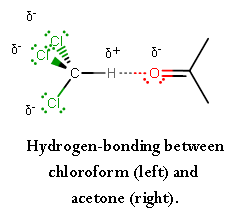
Due to the THREE chlorine atoms on chloroform, the carbon becomes
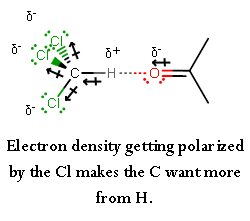
This allows the
The result of this is that we go from acetone-acetone dipole-dipole interactions and chloroform-chloroform dipole-dipole interactions to hydrogen-bonding between each other.
It turns out that this hydrogen-bonding happens to be stronger the original dipole-dipole forces, so this shows NEGATIVE DEVIATION from Raoult's law.
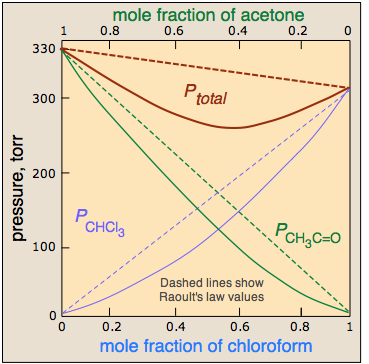
That is, it has a total vapor pressure lower than predicted. This means the volume of the mixture is smaller than the volume we expect if volumes were perfectly additive.

