Answer:
It is due to anatomy of retina of human eye.
Explanation:
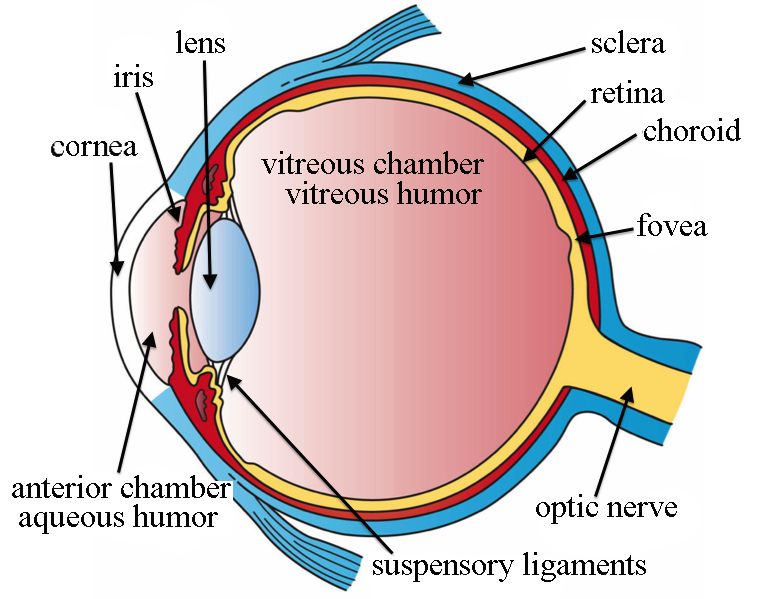
The picture above details the anatomy of eye. The main parts responsible for accommodation of eye and focusing the image of the object which is being viewed are: Cornea, Iris, lens, Suspensory ligaments, Choroid, Ciliary muscles attached to the lens (not shown in the picture), on the left hand side.
A sharp image is formed by the lens system on the photosensitive part of eye called Retina, located at the back of eye; towards the right side of the picture.
The second picture appearing below shows the Retinal anatomy in detail.
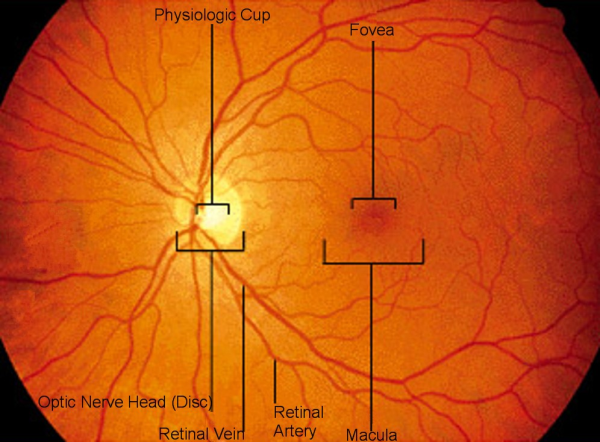
The retina contains two types of photoreceptors (neurons). These are called rods and cones. Visual information received by the photoreceptors is translated to neural signals by the nerves. These signals are carried to our brain via optic nerve, where these are interpreted as our vision.
The optic nerve is devoid of any photoreceptors, as such it corresponds with our natural blind spot.
Cones are highly sensitive to light and color information. These distinguish fine detail which is why they are denser in the central retina.
On the other hand rods do not detect color and are sensitive to dim light, movement and shapes. These are about one thousand times more sensitive than cones.
Next picture below shows concentration of rod and cone photoreceptors in the various parts of retina.
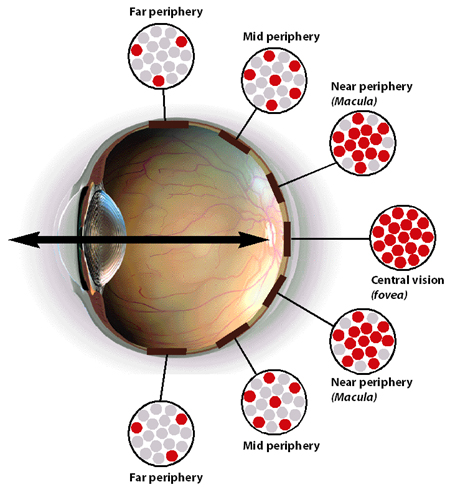
- Central part which consists of Fovea Centralis, often regarded as the most important part of the eye. It is a small dimple in the middle of retina, 0.3 mm diameter rod-free area is packed with cones only, shown as red dots. This is central part of macula.
This part provides our highest resolution vision in terms of light and color.
- Macula, which is responsible for near peripheral vision is also packed with cone photoreceptors, however there are rods in this region as well, as shown by mix of red and gray dots. We use our macular vision to read a book, watch television or work on a computer.
Macula provides about 18 degrees of clear vision. Anything outside of this narrow 18 degrees is not focused. We can see things in our periphery, but these are not clear.
- The periphery of the retina has the highest concentration of rod photoreceptors and very few cone photoreceptors as shown in the picture by mix of red and gray dots. Observe that number of gray dots increases and of red decreases.
With our peripheral vision, which is beyond macular vision, we pick up movements and shapes. This vision is better at night under low light conditions.
While crossing a street at a busy intersection; we are focused ahead so as not to collide with oncoming pedestrians. However, if a car approaches from the side, we pick up its movement which allows us to move out of the way.
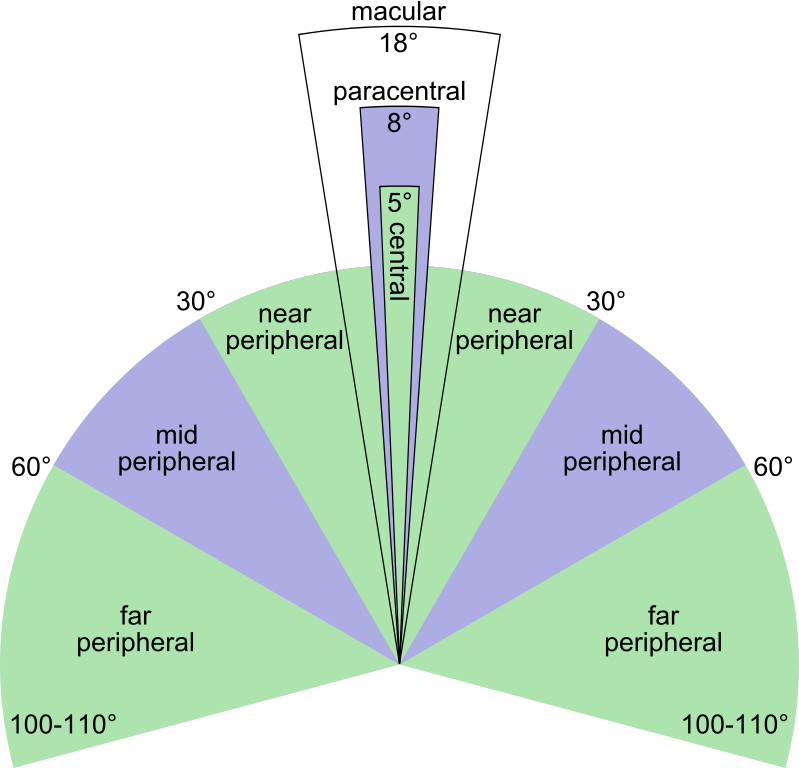
The near absence of cones and abundance of rods in the outer part of retina is the reason for our not getting color or detailed information from our peripheral vision.
Make the internet a better place to learn
More great answers
Answer:
It is due to anatomy of retina of human eye.
Explanation:

The picture above details the anatomy of eye. The main parts responsible for accommodation of eye and focusing the image of the object which is being viewed are: Cornea, Iris, lens, Suspensory ligaments, Choroid, Ciliary muscles attached to the lens (not shown in the picture), on the left hand side.
A sharp image is formed by the lens system on the photosensitive part of eye called Retina, located at the back of eye; towards the right side of the picture.
The second picture appearing below shows the Retinal anatomy in detail.

The retina contains two types of photoreceptors (neurons). These are called rods and cones. Visual information received by the photoreceptors is translated to neural signals by the nerves. These signals are carried to our brain via optic nerve, where these are interpreted as our vision.
The optic nerve is devoid of any photoreceptors, as such it corresponds with our natural blind spot.
Cones are highly sensitive to light and color information. These distinguish fine detail which is why they are denser in the central retina.
On the other hand rods do not detect color and are sensitive to dim light, movement and shapes. These are about one thousand times more sensitive than cones.
Next picture below shows concentration of rod and cone photoreceptors in the various parts of retina.

- Central part which consists of Fovea Centralis, often regarded as the most important part of the eye. It is a small dimple in the middle of retina, 0.3 mm diameter rod-free area is packed with cones only, shown as red dots. This is central part of macula.
This part provides our highest resolution vision in terms of light and color.
- Macula, which is responsible for near peripheral vision is also packed with cone photoreceptors, however there are rods in this region as well, as shown by mix of red and gray dots. We use our macular vision to read a book, watch television or work on a computer.
Macula provides about 18 degrees of clear vision. Anything outside of this narrow 18 degrees is not focused. We can see things in our periphery, but these are not clear.
- The periphery of the retina has the highest concentration of rod photoreceptors and very few cone photoreceptors as shown in the picture by mix of red and gray dots. Observe that number of gray dots increases and of red decreases.
With our peripheral vision, which is beyond macular vision, we pick up movements and shapes. This vision is better at night under low light conditions.
While crossing a street at a busy intersection; we are focused ahead so as not to collide with oncoming pedestrians. However, if a car approaches from the side, we pick up its movement which allows us to move out of the way.

The near absence of cones and abundance of rods in the outer part of retina is the reason for our not getting color or detailed information from our peripheral vision.
How do you convert #(5pi)/7# from radians to degree?

Answer:
It is
Explanation:
From
canceling the
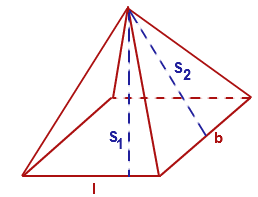
A pyramid has a parallelogram shaped base and a peak directly above its center. Its base's sides have lengths of #8 # and #2 # and the pyramid's height is #9 #. If one of the base's corners has an angle of #(5pi)/12#, what is the pyramid's surface area?
Answer:
Explanation:
Answer:
p= 0.6001
q= 0.399
pq= 313.177 --> 313
Explanation:
We know that our Population X is 653 individuals. Of this population 104 have floppy ears (Xf). We know that p (short) is dominant over q (floppy).
Basic equations for Hardy Weinberg:
p + q = 1
Taking the square root of this number gives us the frequency
q= 0.399
Now we can enter this into p+q=1
This means that p= 1 - q which solves to 0.6001
Now that we now both p and q we can start calculating the frequencies of the genotypes
2pq gives 2 * 0.6001 * 0.399 = 313.177 which gets rounded up to 313
To find the frequency of each genotype we divide the total population by each genotypes' population.
fpp =
fpq =
fqq=
Answer:
The quick answer is that “Like dissolves like”.
Explanation:
Why is this so?
Polar substances tend to dissolve in polar solvents, and nonpolar substances dissolve in nonpolar solvents.
When a solute dissolves in a solvent the individual particles of the solute separate from their neighbours and move between the spaces of the solvent particles.
The solvent particles collide with the solute particles and the intermolecular forces of attraction between solute and solvent particles "hold" the solute particles in the spaces.
There are three steps to the dissolving process:
-
The solvent particles must move apart to make room for solute particles. This process requires energy to overcome forces of attraction between solvent particles. This step is endothermic.
-
The solute particles must separate from their neighbours. This process also requires energy to overcome the forces of attraction between the solute particles. This step is endothermic.
-
When the solute particles move between the solvent particles, the intermolecular forces of attraction between solute and solvent take hold and the particles "snap" back and move closer. This process releases energy. This final step is exothermic.
Consider the process of dissolving a crystal of salt (

A sodium crystal consists of an array of sodium ions (yellow) and chloride ions (green).
Water is a polar solvent: the
When you put the salt in water, the
The ions become solvated (hydrated).
This is an energy-releasing process.
Then, the positive and negative ends of the
The
What if you have a polar liquid such as ethanol,
Will it dissolve in water?
The molecules of ethanol are attracted to each other by H-bonding.
And the molecules of water are attracted to each other by H-bonding.
If you gently add ethanol to water, you may get two layers, in which the less dense ethanol is on the top.

At the interface between the layers, the ethanol molecules can H-bond to the water, and the water molecules can H-bond to the ethanol.
Because the attractions between the molecules are similar, the molecules can mix freely with each other.
Water and ethanol are miscible in all proportions.
All it takes is a gentle swirl, and we have a homogeneous mixture (a solution) of the two substances.
What if you have a nonpolar substance such as hexane?
Will it dissolve in water?
If we add hexane to water, the hexane will float on the top of the water with no apparent mixing.

The attractive forces among the hexane molecules are the relatively weak London dispersion forces.
The attractive forces among the water molecules are the relatively strong H-bonds.
The only attractive forces among the hexane and water molecules are London forces.
Thus, a few hexane molecules will enter the water layer, but the strong attractive forces among the water molecules keeps most of the hexane molecules out.
Similarly, a few water molecules will enter the hexane layer because of the water-hexane London forces.
Water and hexane are immiscible. They do not dissolve in each other.
Finally, will nonpolar substances such as hexane and pentane dissolve in each other?
The attractive forces among both the hexane and the pentane molecules are the relatively weak London dispersion forces.
There is little resistance to a molecule of one compound moving into the other layer.
When the nonpolar pentane molecules move into the nonpolar hexane, London forces are disrupted between the hexane molecules, but new London forces are formed between hexane and pentane molecules.
Because the molecules are so similar, the structure of the solution and the strengths of the attractions between the particles are very similar to the structure and attractions found in the separate liquids.
When these properties are not significantly different in the solution than in the separate liquids, it is largely the natural tendency of a system towards increased disorder (greater entropy) that drives them into solution.
Thus, polarity affects solubility.
If solute and solvent have approximately the same polarity, they will probably form a solution.
“Like dissolves like”: Polar solutes dissolve in polar solvents; nonpolar solutes dissolve in nonpolar solvents.
Answer:
The five major branches of chemistry are organic, inorganic, analytical, physical, and biochemistry. These divide into many sub-branches.
Explanation:
ORGANIC CHEMISTRY
Organic chemistry involves the study of the structure, properties, and preparation of chemical compounds that consist primarily of carbon and hydrogen.
Organic chemistry overlaps with many areas including
- Medicinal chemistry —the design, development, and synthesis of medicinal drugs. It overlaps with pharmacology (the study of drug action).
- Organometallic chemistry — the study of chemical compounds containing bonds between carbon and a metal.
- Polymer chemistry — the study of the chemistry of polymers.
- Physical organic chemistry — the study of the interrelationships between structure and reactivity in organic molecules.
- Stereochemistry — the study of the spatial arrangements of atoms in molecules and their effects on the chemical and physical properties of substances.
INORGANIC CHEMISTRY
Inorganic chemistry is the study of the properties and behaviour of inorganic compounds.
It covers all chemical compounds except organic compounds.
Inorganic chemists study things such as crystal structures, minerals, metals, catalysts, and most elements in the Periodic Table.
Branches of inorganic chemistry include:
-
Bioinorganic chemistry — the study of the interaction of metal ions with living tissue, mainly through their direct effect on enzyme activity.
-
Geochemistry — the study of the chemical composition and changes in rocks, minerals, and atmosphere of the earth or a celestial body.
-
Nuclear chemistry — the study of radioactive substances.
-
Organometallic chemistry — the study of chemical compounds containing bonds between carbon and a metal.
-
Solid-state chemistry — the study of the synthesis, structure, and properties of solid materials.
ANALYTICAL CHEMISTRY
Analytical chemistry involves the qualitative and quantitative determination of the chemical components of substances.
Examples of areas using analytical chemistry include:
-
Forensic chemistry — the application of chemical principles, techniques, and methods to the investigation of crime.
-
Environmental chemistry —the study of the chemical and biochemical phenomena that occur in the environment.It relies heavily on analytical chemistry and includes atmospheric, aquatic, and soil chemistry.
-
Bioanalytical Chemistry — the examination of biological materials such as blood, urine, hair, saliva, and sweat to detect the presence of specific drugs.
PHYSICAL CHEMISTRY
Physical Chemistry —the study of the effect of chemical structure on the physical properties of a substance.
Physical chemists typically study the rate of a chemical reaction, the interaction of molecules with radiation, and the calculation of structures and properties.
Sub-branches of physical chemistry include:
-
Photochemistry — the study of the chemical changes caused by light.
-
Surface chemistry — the study of chemical reactions at surfaces of substances. It includes topics like adsorption, heterogeneous catalysis, formation of colloids, corrosion, electrode processes, and chromatography.
-
Chemical kinetics — the study of the rates of chemical reactions, the factors affecting those rates, and the mechanism by which the reactions proceed.
-
Quantum chemistry — the mathematical description of the motion and interaction of subatomic particles. It incorporates quantization of energy, wave-particle duality, the uncertainty principle, and their relationship to chemical processes.
-
Spectroscopy — the use of the absorption, emission, or scattering of electromagnetic radiation by matter to study the matter or the chemical processes it undergoes.
BIOCHEMISTRY
Biochemistry is the study of chemical reactions that take place in living things. It tries to explain them in chemical terms.
Biochemical research includes cancer and stem cell biology, infectious disease, and cell membrane and structural biology.
It spans molecular biology, genetics, biochemical pharmacology, clinical biochemistry, and agricultural biochemistry.
-
Molecular biology — the study of the interactions between the various systems of a cell, such as the different types of DNA, RNA, and protein biosynthesis.
-
Genetics — the study of genes, heredity, and variation in living organisms.
-
Pharmacology — the study of mechanisms of drug action and the influence of drugs on an organism.
o Toxicology —a sub-branch of pharmacology that studies the effects of poisons on living organisms. -
Clinical biochemistry — the study of the changes that disease causes in the chemical composition and biochemical processes of the body.
-
Agricultural biochemistry — the study of the chemistry that occurs in plants, animals, and microorganisms.
Thus, although there are FIVE main branches of chemistry, there are many sub-branches.
There is a huge overlap between Chemistry and Biology, Medicine, Physics, Geology, and many other disciplines.
Chemistry really is THE CENTRAL SCIENCE.
Answer:
The wavelength decreases as the frequency increases.
Explanation:
The wavelength
The frequency
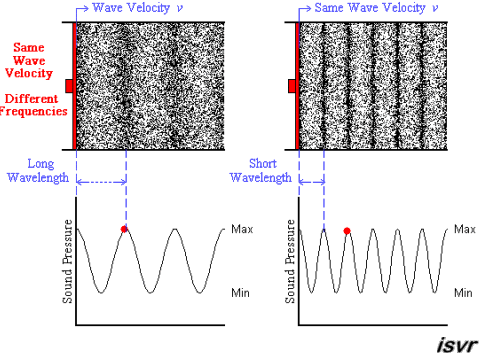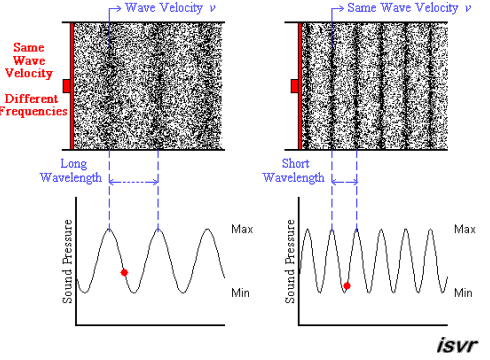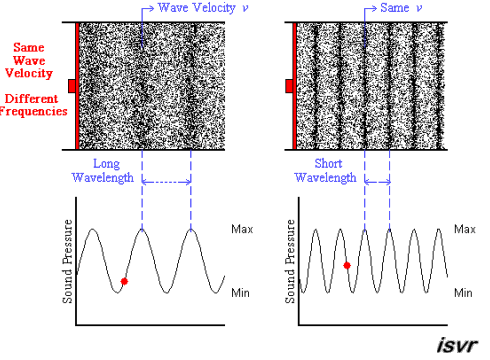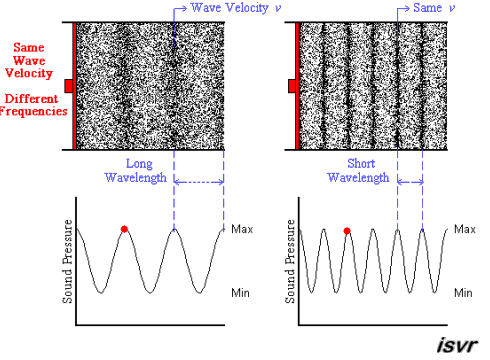
Above we see two sound waves travelling through space at the same velocity
The red dot follows the amplitude of the wave as it goes by.
The frequency of oscillation of the dot is the frequency
You can see that the wavelength is halved when the frequency is doubled.
The frequency
Since
Answer:
The overall (unbalanced) chemical equation for cellular respiration is:
Explanation:
The balanced equation is
The equation expressed in words would be:
The equation is formulated by combining the three following processes into one equation:
-
Glycolysis — the breakdown of the form of a glucose molecule into two three-carbon molecules i.e. pyruvate (pyruvic acid).
-
The Tricarboxylic Acid Cycle or Krebs Cycle — the three-carbon pieces are pulled apart bit by bit to release the energy stored in those covalent bonds. This is where most of the
#"CO"_2# is formed. -
The Electron Transport Chain and Oxidative Phosphorylation — this sequence requires the
#"O"_2# and produces most of the energy. This energy comes in the form of#"ATP"# , or adenine triphosphate.
Answer:
You divide the final volume by the initial volume.
Explanation:
EXAMPLE 1:
What is the dilution factor if you add a 0.1 mL aliquot of a specimen to 9.9 mL of diluent?
Solution:
You have diluted the sample by a factor of 100.
The dilution factor is often used as the denominator of a fraction.
For example, a
EXAMPLE 2:
How would you make 500 mL of a 1:250 dilution?
Solution:
Pipet 2.00 mL of your stock solution into a 500 mL volumetric flask.
Add diluent to the mark on the flask (you will have added about 498 mL of water).
You now have a 1:250 dilution of your original solution.
Answer:
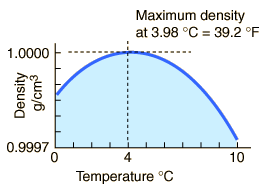
The maximum density of water occurs at 4 °C because, at this temperature two opposing effects are in balance.
Explanation:
In ice, the water molecules are in a crystal lattice that has a lot of empty space.
When the ice melts to liquid water, the structure collapses and the density of the liquid increases.
At temperatures well above freezing, the molecules move faster and get further apart. The density decreases as temperature increases.
At temperatures near 0 °C, the water still contains many ice-like clusters. These clusters are free to move relative to each other, so water is still liquid.
The clusters still have empty spaces, so they decrease the density of the liquid.
The molecules of the water are closer together, and this increases the density of the liquid.
Now, let's cool the water.
As the temperature of warm water decreases, the water molecules slow down and the density increases.
At 4 °C, the clusters start forming.
The molecules are still slowing down and coming closer together, but the formation of clusters makes the molecules be further apart.
Cluster formation is the bigger effect, so the density starts to decrease.
Thus, the density of water is a maximum at 4 °C.
What is dna?

Answer:
Deoxyribonucleic Acid is the meaning of it. It is a nucleic acid that is the carrier of genetic information.
Explanation:
DNA are the letters of deoxyribonucleic acid.
All life on earth uses this nucleic acid as the genetic code.
A nucleic acid is a polynucleotide. A polynucleotide consists of three basic units: a phosphate group, a 5 carbon sugar (pentose), and a nitrogenous base. The five carbon sugar is deoxyribose. Since a polynucleotide chain, the phosphate and deoxyribose units are repetitive, the variation is provided by the nitrogenous bases.
There are four bases: adenine, guanine, cytosine, and thymine.
Both adenine and guanine are purines which have a double ring structure. Cytosine and thymine are pyramidines which consist of a single ring structure.
The DNA molecule is double helix, a spiral shaped ladder. The upright or backbone of the ladder is made of alternating pentose and phosphate groups held together by covalent bonds. The rungs or steps of the ladder consist of the bases. These bases are joined to the pentose sugars with covalent bonds. Adenine pairs with thymine using two hydrogen bonds and cytosine pairs with guanine using three hydrogen.
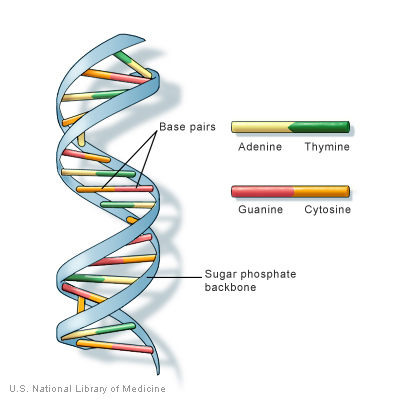
The genetic code is determined by the linear sequence of the bases.
For example the sequence of adenine guanine thymine does not carry the same message as guanine thymine adenine.
The code is arranged in triplet form which codes for RNA which in turn codes for amino acids which form the basis of proteins.


